Mitigating Risks of Compounded Drugs Through Surveillance
FDA’s Compounding Incidents Program aims to help protect the public against poor quality compounded drugs through surveillance and review of complaints, which include adverse event and product quality reports. As the Incidents Program identifies risks related to compounded drugs, it takes action to mitigate these risks. The program also communicates to raise stakeholder and public awareness about risks related to human drug compounding.
- An adverse drug experience is defined as any adverse event associated with the use of a drug in humans, whether or not the event is considered drug-related, including those occurring:
- in the course of the use of a drug product in professional practice
- from drug overdose whether accidental or intentional
- from drug abuse or drug withdrawal
- any failure of expected pharmacological action
- Other complaints cover a wide range of issues beyond adverse events. Examples include:
- product quality issues (such as sub- or super-potency, contamination, particulate matter, or pH issues)
- insanitary conditions within a facility
- compounders making false or misleading claims about their drugs
- compounding of ineligible bulk drug substances
Why is it important to report adverse events and other complaints?
Adverse event and other complaint reporting associated with compounded human drug products is a critical mechanism for identifying potential quality problems that may be associated with a particular drug or drug component and which may have been caused by conditions or processes at a facility where the drug or its components were made or handled.
The Compounding Incidents Program uses reports to distinguish such cases from cases of medication error, hospital or clinic procedural problems, or quality issues associated with ingredients (e.g., active pharmaceutical ingredients (APIs), excipients) so that appropriate action can be considered. For example, several reports of adverse events in patients who received compounded drug products from the same facility may be a signal of a quality issue resulting from a deficiency in the firm’s processes. However, if several different facilities report adverse events in patients who received compounded drug products that contained the API, this may suggest a quality problem associated with the API used in the compounded drug products.
How many reports does the Compounding Incidents Program receive?
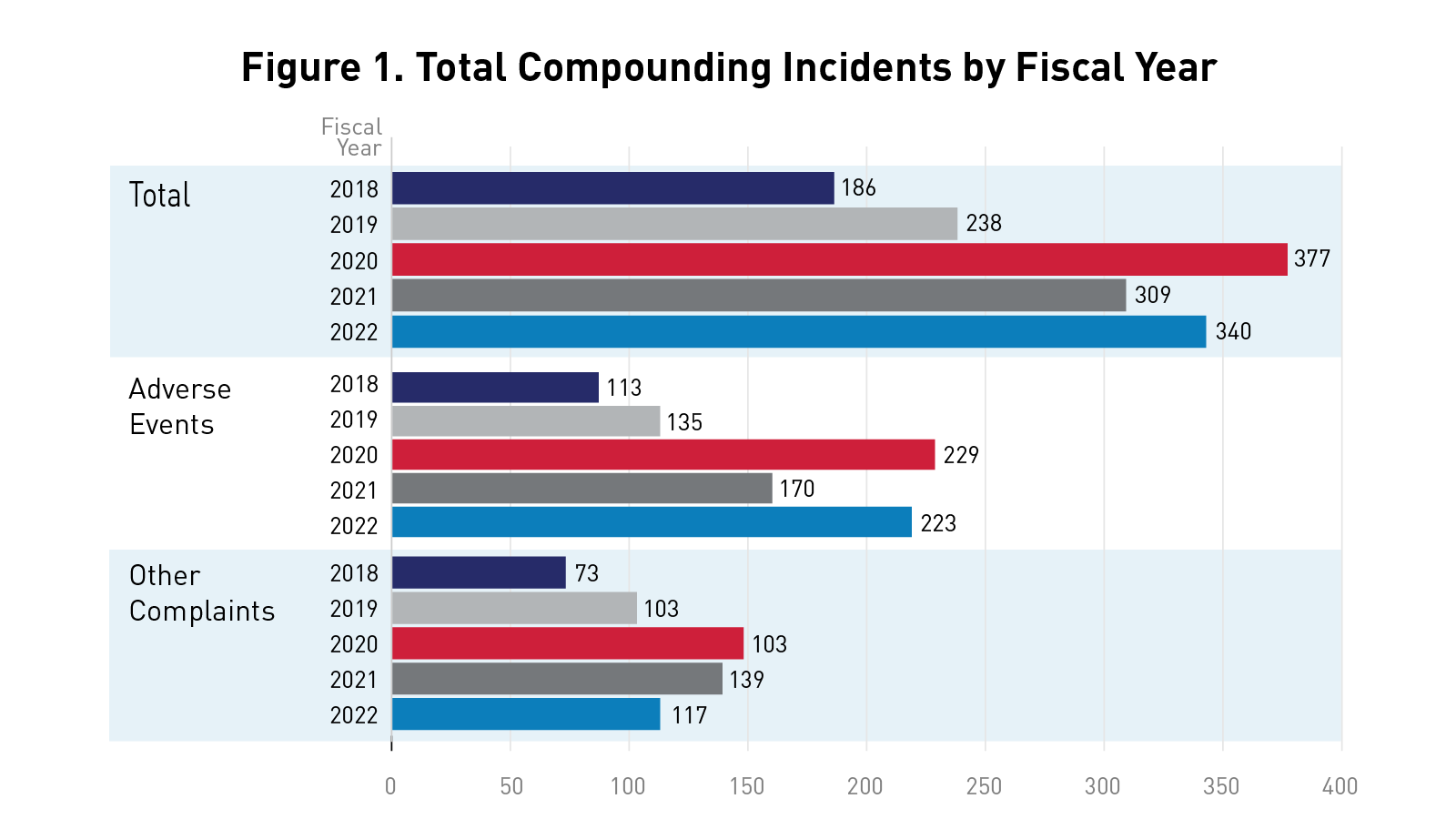
Figure 1 below highlights the total number of incidents (adverse events and other complaints) reported to FDA’s Compounding Incidents Program by fiscal year.
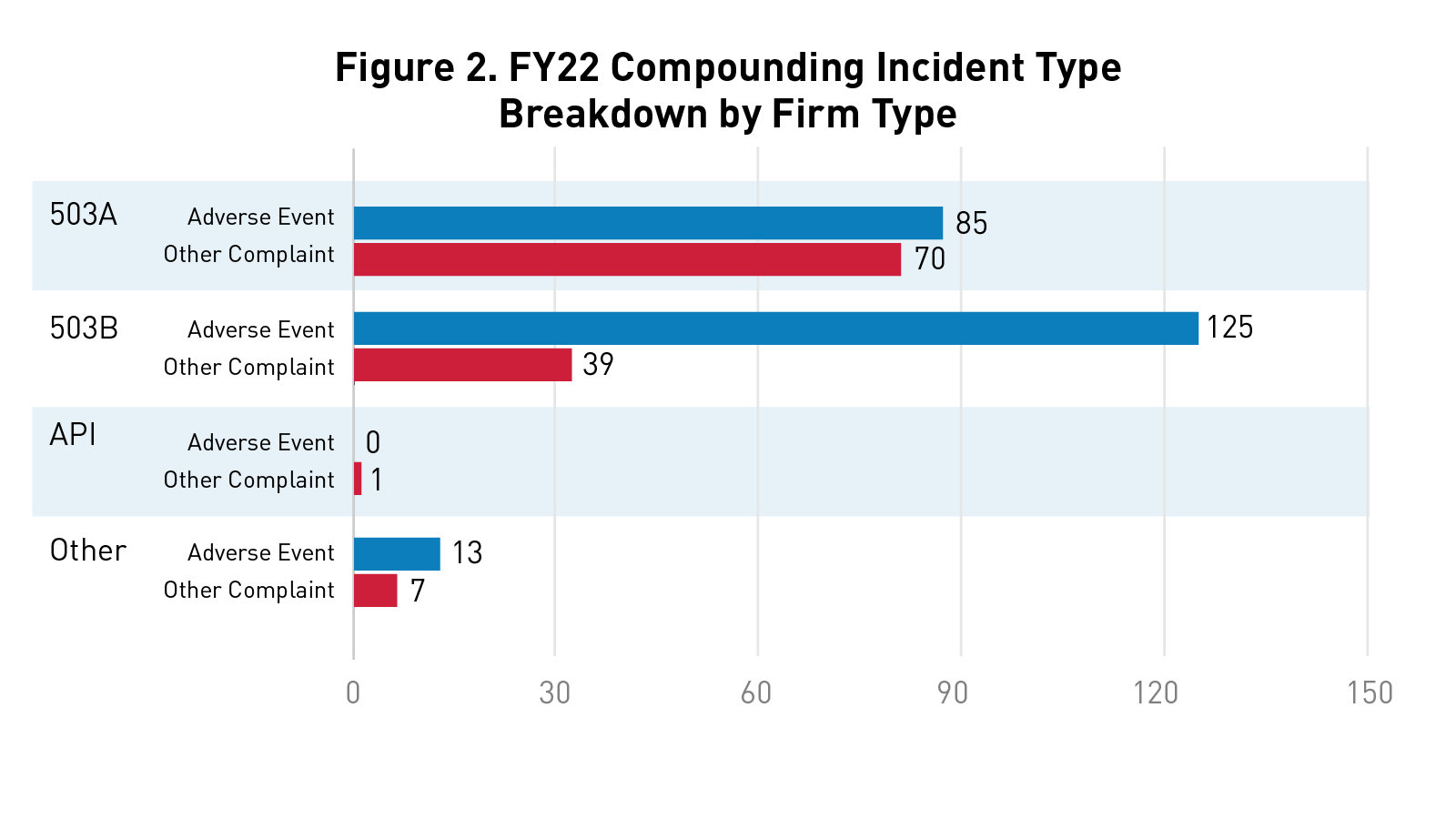
Figure 2 below highlights the number of adverse events and other complaints received by firm type in fiscal year 2022.
API includes active pharmaceutical ingredient (API) manufacturers, distributors, or repackers. Other firm types include unknown firms and marketing firms.
Who reports incidents related to compounding to FDA?
The Compounding Incidents Program receives compounding incidents from consumers, healthcare professionals, compounders, states, and others. Some sources of the reports include:
- Voluntary MedWatch reports from consumers, and healthcare professionals
- 503A compounders
- 503B outsourcing facilities
- Outsourcing facilities must electronically report to FDA all serious and unexpected adverse drug experiences associated with the use of their compounded prescription drug products within 15 calendar days of receipt.
- For more information, please see Guidance for Industry, Adverse Event Reporting for Outsourcing Facilities Under Section 503B of the Federal Food, Drug, and Cosmetic Act.
- Other FDA component offices such as the Office of Regulatory Affairs, which conducts agency inspections and the Division of Drug Information
- State regulatory authorities, such as state boards of pharmacy
- Other federal agencies, such as the Center for Disease Control and Prevention and Drug Enforcement Agency
- Professional organizations
Information from consumers, healthcare professionals, and compounders on issues related to compounded products helps FDA to better identify trends, locate the source of the issue through investigation, and take action as necessary. However, FDA cannot take action without the knowledge of such issues.
What is the process for reviewing reports submitted to FDA related to compounding?
All incidents received are logged into a database and reviewed by the Compounding Incidents Program for appropriate action. Each incident is assigned to a consumer safety officer who researches and investigates the incident, looking for any signals of larger safety issues. FDA may contact the reporter of the incident directly to obtain missing or additional information that will aid the evaluation.
The Incident Coordination Group discusses incidents to determine next steps as necessary. The Incident Coordination Group is a multi-office collaboration of stakeholders in the agency with expertise in various areas such as manufacturing and pharmaceutical quality, drug information, and pharmacovigilance.
What actions has the Compounding Incidents Program taken?
The Compounding Incidents Program continuously adapts investigation and actions to cases as the program receives incidents of varying topics and complexities. Multiple actions may also be taken in response to an incident with many complex factors influencing the outcome. Examples of the actions that may be taken include:
- Inspection, which may lead to regulatory actions, such as warning letters, untitled letters, recalls, or other actions
- Sample collection and testing at FDA laboratories
- Referral or notification to other FDA centers or offices for appropriate action if an issue spans product areas
- Notification to and/or a request for information from other government agencies or state regulatory authorities
- In FY2021, the program issued 72 notification letters to 19 states. State regulatory authorities in receipt of these letters included boards of pharmacy, boards of medicine, boards of nursing, boards of chiropractic examiners, physician assistant board, and departments of health.
- Notification of professional organizations, such as the National Association of Boards of Pharmacy and the Federation of State Medical Boards, of broader issues identified related to compounding.
- Public communications such as Compounding Risk Alerts.
- Developed in 2017, Compounding Risk Alerts inform health care professionals, compounders, and patients about adverse events, drug quality issues, and other issues related to compounded drugs that pose a risk to public health so that practitioners can more effectively protect patients from poor-quality compounded medicines.
- Incidents can also be a driving force for revision or development of policies or guidance documents.
Want to know more?
An article by the Compounding Incidents Program and published in the American Journal of Health-System Pharmacy provides additional insight into the history and processes of the Compounding Incidents Program.
A pharmacist-driven Food and Drug Administration incident surveillance and response program for compounded drugs, American Journal of Health-System Pharmacy (AJHP), April 23, 2021
source and quoted from Mitigating Risks of Compounded Drugs Through Surveillance | FDA

No comments:
Post a Comment