Human Medications, Human Drugs, Animal Medications, Animal Drugs, Pharmacy law, Pharmaceutical law, Compounding law, Sterile and Non Sterile Compounding 797 Compliance, Veterinary law, Veterinary Compounding Law; Health Care; Awareness of all Types of Compounding Issues; Pharmacy Benefit Managers (PBMs), Outsourcing Facilities Food and Drug Administration and Compliance Issues
Friday, March 26, 2021
Thursday, March 25, 2021
Memorandum of Understanding Addressing Certain Distributions of Compounded Drugs
FDA announced in October 2020 the standard Memorandum of Understanding Addressing Certain Distributions of Compounded Human Drug Products became available for signature by the states. This MOU is an agreement between state boards of pharmacy or other state agencies and FDA. The MOU addresses interstate distribution of inordinate amounts of compounded drugs and complaint investigation by a state regulator relating to compounded drugs distributed outside the state.
FDA developed the MOU in consultation with the National Association of Boards of Pharmacy (NABP), as described in section 503A of the Federal Food, Drug, and Cosmetic Act (FD&C Act). The MOU is a key public health protection in the law and is anticipated to help enhance communication and maximize federal and state resources for oversight of compounded drugs produced by traditional pharmacies.
Reducing the risks associated with compounded drugs
States, along with FDA, play a vital role in helping to reduce the risks associated with compounded drugs, while preserving appropriate patient access. For example, if a compounder distributes compounded drugs to multiple states, it can be difficult to gather information about adverse drug experiences and quality issues associated with those drugs, connect them to the compounder and take coordinated action to address a potentially serious public health problem. Close collaboration among states, and between states and the federal government may help prevent serious and widespread problems by helping to better identify adverse drug experiences and drug quality concerns across the country.
Information sharing network
FDA has entered into a cooperative agreement with NABP to establish an information sharing network available to the states. Through this network, FDA expects that states will have the option to collect, assess and review information about pharmacies in their states and share information with FDA as outlined in the MOU.
This tool is intended to provide an option for states and potentially reduce the resource burden of reporting under the MOU for states that choose to use it.
Expected benefits of the information sharing network:
- Information access: facilitate information sharing between state regulators and FDA on the distribution of compounded drugs interstate and complaints related to drugs compounded in the state and distributed outside the state
- Facilitate collaboration: enhance state and federal oversight and help regulators focus limited resources on compounders that present the greatest risk
- Streamlined identification: enable state regulators to identify pharmacies that distribute an inordinate amount of compounded drugs interstate and report that information to FDA
Complaint investigations
Under the MOU, states agree to investigate complaints about adverse drug experiences and drug quality issues related to drugs compounded at pharmacies within the state and distributed outside the state. States then notify FDA about adverse drug experience and drug quality issues relating to a drug compounded at a pharmacy, if serious, or by a physician as soon as possible, but no later than five business days after the state receives the complaint. This timeframe will facilitate collaboration between states and FDA on serious issues that have the potential to affect patients in multiple states.
Inordinate amounts and the 50% threshold for information sharing
Under the MOU, states agree to share certain information with FDA about pharmacies that distribute more than 50% of their compounded drug prescription orders interstate (see III.b.1 of MOU). The MOU, however, does not place a limit on the distribution of compounded drugs interstate by a pharmacy located in a state that has signed the MOU.
States that sign the MOU also agree to report to FDA if they become aware of a physician who is distributing compounded drugs interstate.
5% statutory limit on distributing compounded drugs out of the state
Section 503A of the FD&C Act limits distribution of compounded drugs outside the state by a licensed pharmacist, pharmacy or physician located in a state that has not signed the MOU to 5% of its total prescription orders dispensed or distributed.
Timeframe for signature
States may sign the MOU at any time. FDA is providing a period of one year, which concludes on October 27, 2021, for states to consider signing the MOU before it intends to enforce the 5% limit in section 503A of the FD&C Act in states that have not signed the MOU. This timeframe should correspond to a full legislative cycle for most states and allows time for states to modify their laws and regulations, if necessary.
Products not covered by the MOU
Products not covered by the MOU include:
- drugs intended for veterinary use
- repackaged drug products
- radiopharmaceuticals
- biological products subject to licensure under section 351 of the Public Health Service Act
- drugs compounded by outsourcing facilities under section 503B of the FD&C Act
Questions
See Memorandum of Understanding Addressing Certain Distributions of Compounded Drugs Questions and Answers for more information. Please email questions regarding the MOU to compounding@fda.hhs.gov.
Additional information
Tuesday, March 23, 2021
Department of Justice
Pharmacist Charged in $4 Million Health Care Fraud and Kickback Scheme
A New York man was arrested today for his role in a conspiracy to commit health care fraud and to pay kickbacks and bribes to customers for expensive prescription orders in connection with more than $4 million in Medicare and Medicaid reimbursements.
According to an indictment returned by a federal grand jury in the Eastern District of New York, Robert John Sabet, 44, of Brooklyn, was the owner of Brooklyn Chemists in Gravesend, Brooklyn, and Lucky Care Pharmacy in Flushing, Queens. Since September 2016, Sabet allegedly conspired to bill Medicare and Medicaid for expensive prescription drugs that were not eligible for reimbursement because, among other reasons, they were not needed or not dispensed. Sabet also allegedly conspired to pay kickbacks and bribes to customers to convince them to fill prescriptions at his pharmacies, and to pay customers cash in exchange for the ability to bill Medicare and Medicaid for over-the-counter health care-related products on their behalf.
In December 2020, Sabet allegedly wired nearly $100,000 from Lucky Care’s bank accounts to an automobile dealership to pay for a luxury car. Investigators conducted a search warrant at Sabet’s home at the time of his arrest and seized a 2020 Porsche Taycan worth over $250,000, as well as cash and luxury goods.
Sabet is charged with conspiracy to commit health care fraud, conspiracy to defraud the United States by paying kickbacks and bribes in connection with the provision of health care services, and unlawfully spending the proceeds of his fraud. The defendant is scheduled for his initial court appearance today before U.S. Magistrate Judge Ramon E. Reyes, Jr. of the U.S. District Court for the Eastern District of New York. If convicted, he faces a maximum penalty of 10 years in prison for conspiracy to commit health care fraud, five years in prison for conspiracy to pay kickbacks and bribes, and 10 years in prison for unlawful spending. A federal district court judge will determine any sentence after considering the U.S. Sentencing Guidelines and other statutory factors.
Acting Assistant Attorney General Nicholas L. McQuaid of the Justice Department’s Criminal Division; Acting U.S. Attorney Seth D. DuCharme of the Eastern District of New York; Special Agent in Charge Scott J. Lampert of the Health and Human Services Office of Inspector General (HHS-OIG), New York Regional Office; Special Agent in Charge Jonathan D. Larsen of IRS-Criminal Investigation (IRS-CI), New York; and Acting Medicaid Inspector General Frank T. Walsh Jr. of the New York State Office of the Medicaid Inspector General (OMIG) made the announcement.
HHS-OIG, IRS-CI, and OMIG are investigating the case.
Trial Attorney Miriam L. Glaser Dauermann of the Justice Department’s Fraud Section is prosecuting the case.
An indictment is merely an allegation and all defendants are presumed innocent until proven guilty beyond a reasonable doubt in a court of law.
| 03/23/2021 | 03/05/2021 | Panther James LLC | Division of Human and Animal Food Operations East VI | Juice HACCP/CGMP for Foods/Adulterated/Insanitary Conditions | ||
| 03/23/2021 | 03/10/2021 | Aman Kapoor In | Division of Northern Border Imports | Foreign Supplier Verification Program (FSVP) | ||
| 03/23/2021 | 03/18/2021 | DEPQ Internacional S. de R.L de C.V. | Center for Drug Evaluation and Research | Finished Pharmaceuticals/Unapproved New Drug/Misbranded/Adulterated | ||
| 03/23/2021 | 01/25/2021 | PYRLess Group, LLC dba Dr. Fitt | Center for Food Safety and Applied Nutrition | Unapproved and Misbranded Products Related to Coronavirus Disease 2019 (COVID-19) | ||
| 03/22/2021 | 03/15/2021 | Honest Globe, Inc. | Division of Pharmaceutical Quality Operations IV | CGMP/Finished Pharmaceuticals/Adulterated | ||
| 03/22/2021 | 03/18/2021 | BioLyte Laboratories, LLC | Division of Pharmaceutical Quality Operations III | CGMP/Finished Pharmaceuticals/Adulterated | ||
| 03/19/2021 | 03/19/2021 | Vapor Outlet Inc d/b/a Union Hills Vape Shop | Center for Tobacco Products | Family Smoking Prevention and Tobacco Control Act/Adulterated/Misbranded | ||
| 03/19/2021 | 03/19/2021 | Vapor Tech Hawaii, Inc. | Center for Tobacco Products | Family Smoking Prevention and Tobacco Control Act/Adulterated/Misbranded | ||
| 03/19/2021 | 03/19/2021 | The Vaporium | Center for Tobacco Products | Family Smoking Prevention and Tobacco Control Act/Adulterated/Misbranded | ||
| 03/16/2021 | 03/10/2021 | Foshan Biours Biosciences Co., Ltd. | Center for Drug Evaluation and Research | CGMP/Finished Pharmaceuticals/Adulterated |
| 03/16/2021 | 03/10/2021 | Dibar Nutricional S. de R.L. de C.V. | Center for Drug Evaluation and Research | CGMP/Finished Pharmaceuticals/Adulterated | ||
| 03/16/2021 | 03/11/2021 | Albek de Mexico S.A. de C.V. | Center for Drug Evaluation and Research | Finished Pharmaceuticals/Unapproved New Drug/Misbranded/Adulterated | ||
| 03/16/2021 | 03/16/2021 | Little Town Vaping d/b/a Swamp Vapor Lafayette | Center for Tobacco Products | Family Smoking Prevention and Tobacco Control Act/Adulterated/Misbranded | ||
| 03/16/2021 | 03/16/2021 | Little Town Vaping d/b/a Swamp Vapor | Center for Tobacco Products | Family Smoking Prevention and Tobacco Control Act/Adulterated/Misbranded | ||
| 03/16/2021 | 03/16/2021 | Little Town Vaping d/b/a Swamp Vapor New Iberia | Center for Tobacco Products | Family Smoking Prevention and Tobacco Control Act/Adulterated/Misbranded | ||
| 03/15/2021 | 03/12/2021 | Ravenscroft Apothecary, Inc. DBA Ravenscroft Escentials | Center for Drug Evaluation and Research | Unapproved and Misbranded Products Related to Coronavirus Disease 2019 (COVID-19) |
- As part of the FDA’s effort to protect consumers, the agency issued a warning letter jointly with the Federal Trade Commission to PYRLess Group, LLC dba Dr. Fitt for selling unapproved products with fraudulent COVID-19 claims. The FDA requested that the company take immediate action to cease the sale of any unapproved and misbranded products for the treatment or prevention of COVID-19. Consumers concerned about COVID-19 should consult with their health care providers.
FDA Warns Companies Illegally Selling Over-the-Counter CBD Products for Pain Relief
Products Listing CBD as Inactive Ingredient Cited for Unapproved Drug and Misbranding Violations
The U.S. Food and Drug Administration has issued warning letters to two companies for selling products labeled as containing cannabidiol (CBD) in ways that violate the Federal Food, Drug, and Cosmetic Act (FD&C Act). Specifically, the warning letters address the illegal marketing of unapproved drugs labeled as containing CBD. The FDA has not approved any over-the-counter (OTC) drugs containing CBD, and none of these products meet the requirements to be legally marketed without an approved new drug application. The letters explain that, as CBD has known pharmacological effects on humans, with demonstrated risks, it cannot be legally marketed as an inactive ingredient in OTC drug products that are not reviewed and approved by the FDA. Additionally, the letters cite substandard manufacturing practices, including failure to comply with current good manufacturing practices.
“The FDA continues to alert the public to potential safety and efficacy concerns with unapproved CBD products sold online and in stores across the country,” said FDA Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D. “It’s important that consumers understand that the FDA has only approved one drug containing CBD as an ingredient. These other, unapproved, CBD products may have dangerous health impacts and side effects. We remain focused on exploring potential pathways for CBD products to be lawfully marketed while also educating the public about these outstanding questions of CBD’s safety. Meanwhile, we will continue to monitor and take action, as needed, against companies that unlawfully market their products — prioritizing those that pose a risk to public health.”
The FDA issued warning letters to:
The products that are the subject of the warning letters issued today have not gone through the FDA drug approval process and are considered unapproved new drugs. There has been no FDA evaluation of whether these unapproved drug products are effective for the uses manufacturers claim, what an appropriate dose might be, how they could interact with FDA-approved drugs or other products or whether they have dangerous side effects or other safety concerns.
The FDA has previously sent warning letters to other companies illegally selling unapproved CBD products that claimed to prevent, diagnose, mitigate, treat or cure various diseases, in violation of the FD&C Act.
Under the FD&C Act, any product intended to diagnose, cure, mitigate, treat or prevent a disease, and any product (other than a food) that is intended to affect the structure or function of the body of humans, is a drug. OTC drugs must be approved by the FDA or meet the requirements for marketing without an approved new drug application under federal law, including drug products containing CBD, regardless of whether CBD is represented on the labeling as an active ingredient or an inactive ingredient.
The FDA has not approved any CBD-containing drug products other than one prescription drug for the treatment of seizures associated with tuberous sclerosis complex, Lennox-Gastaut syndrome and Dravet syndrome in human patients.
The FDA has requested written responses from these companies within 15 working days stating how they will address these violations or providing their reasoning and supporting information as to why they believe these products are not in violation of the law. Failure to adequately address the violations promptly may result in legal action, including product seizure and/or injunction.
Additional Resources:
Friday, March 19, 2021
South Florida men accused of pushing prescription drugs for kickbacks By RON HURTIBISE SOUTH FLORIDA SUN SENTINEL | MAR 19, 2021 AT 7:00 AM Compounding pharmacies create prescription creams by blending two or more drugs to form a compound that's otherwise not available from drug makers. Compounding pharmacies create prescription creams by blending two or more drugs to form a compound that's otherwise not available from drug makers. (Seasontime/Shutterstock) Two South Florida businessmen have agreed to pay $4 million to settle federal charges that they engaged in schemes to overcharge public health insurers for drugs that patients didn’t order or want. The kickback scheme enriched a long list of defendants who colluded to obtain and fill prescriptions for expensive compounded drugs and then submitted reimbursement claims to Medicare and armed-services insurer TRICARE that generated millions of dollars, according to a 2015 federal lawsuit. Advertisement Thirty-six other Florida companies named as defendants in the case, including pharmacies, health providers and marketers, previously settled charges filed under the federal False Claims Act by the United States Attorney’s Office for the Middle District of Florida. RELATED: Were you victimized by South Florida's most depraved scams? » The remaining defendants, Jack Lee Stapleton, 75, of Gulf Stream, and Jack Hunter Stapleton, 33, of Fort Lauderdale, were accused of using telemarketers to persuade patients to accept expensive compounded drugs, then paying telemedicine providers to prescribe those drugs without ever seeing the patients or conducting any meaningful medical examinations. The Stapletons, who operated numerous companies with various names in South Florida, sent the prescriptions to compounding pharmacies that agreed to pay the men half of whatever they were reimbursed, the lawsuit claimed. They focused on compounded drugs — custom-made combinations of two or more drugs — that were not mass-produced and could be invoiced for thousands of dollars per prescription. “Kickback arrangements undermine confidence in our health care system,” said Brian M. Boynton, acting assistant attorney general of the Department of Justice’s civil division, in a news release announcing the settlement. “This case demonstrates how kickback schemes often result in the provision of medically unnecessary services at the taxpayer’s expense.” Advertisement The release stated that the Stapletons’ decision to pay $4 million resolved claims that were only allegations, adding, “There has been no determination of liability.” According to the suit, telemarketers would call patients with diabetes, cardiovascular conditions and other diseases to get their consent to obtain prescriptions from associated doctors. The products usually involved creams related to treatments for pain, wounds, scars and similar conditions. RELATED: How 'outright lies' duped victims of health insurance 'scam' » The marketers persuaded physicians to prescribe compounds with high doses of the corticosteroid Fluticasone and the anti-inflammatory drug Flurbiprofen, the complaint states, because they could bill Medicare and TRICARE thousands of dollars more than for compounds made with Ketoprofen, which is as effective as Flurbiprofen but costs 72% less. One of the pharmacies received $13,497,447 for compounds containing Flurbiprofen, the suit said. If one of the participating pharmacies was unable to produce a compounded drug, it would transfer the prescription to another pharmacy and both would split the proceeds, the suit states. RELATED: How a ‘shady’ insurance lawyer sucked homeowners into his alleged ripoff scheme » The scheme ran into complications when patients began complaining to their insurers about being contacted by marketers who worked for one of the Stapletons’ companies, C.V. McDowell, and being urged to take prescriptions that they didn’t want, need or request. One patient complained that “C.V. McDowell wouldn’t stop calling her about a refill she did not want (she never wanted or requested the first one) for a wound she does not have.” Morning Update Newsletter Weekdays Start your day with the top stories in South Florida. ENTER YOUR EMAIL ADDRESS Patients became suspicious, the suit stated, because they were also told they didn’t have to remit a co-pay. According to the lawsuit, the companies involved in the scheme absorbed the patients’ co-payments to induce them into accepting the prescriptions. Waiving or reducing co-payments violates laws prohibiting kickbacks. RELATED: Moving companies with no trucks or movers can break your bank account, your stuff and your heart. Here’s how to avoid them » The scheme was exposed by Dwayne Thornton, co-plaintiff of the government’s lawsuit and former vice president of operations for one of the companies, Bradenton-based Soothe Pharmacy, charged in the suit. Thornton filed his complaint after noticing the illegal practices, fearing he would be criminally charged, and failing to persuade his bosses and principals of other companies involved in the scheme to stop illegal practices. Because the U.S. Attorney’s Office took over the case, Thornton will receive an undetermined portion of the $4 million settlement, the Department of Justice said in its news release. Ron Hurtibise Ron Hurtibise South Florida Sun Sentinel CONTACT Ron Hurtibise is a business reporter for the South Florida Sun Sentinel. A Florida resident since 1977, he covers property and health insurance, tourism, the automotive industry, and consumer topics. He is a Florida State University graduate and a former investigative reporter at the Daytona Beach News-Journal. You can call him at 954-356-4071. ADVERTISEMENT LATEST BUSINESS Virtual Broward job fair offered for unemployed hospitality and retail workers 2h Virtual Broward job fair offered for unemployed hospitality and retail workers The red-hot housing market means you’re probably in for a tax increase 11:13 AM The red-hot housing market means you’re probably in for a tax increase Fort Lauderdale cocktail lounge the Wilder changing its stripes, snares chef from Lionfish in Delray Beach 8:48 AM Fort Lauderdale cocktail lounge the Wilder changing its stripes, snares chef from Lionfish in Delray Beach These Cars Are So Loaded It's Hard to Believe They're So Cheap LUXURY SUVS | SEARCH ADS | SPONSORED Play this game for 3 minutes and see why everyone is addicted TOTAL BATTLE - TACTICAL GAME ONLINE | SPONSORED Finally - A perfect mask to breathe freely all day long. SPORTSMASK | SPONSORED All Oklahoma Low Mileage Drivers Should Claim This (Check If You Qualify) SMARTLIFESTYLETRENDS | SPONSORED Oklahoma Homeowners Are Getting a Big Pay Day in March SAVE HOMEOWNERS INSURANCE | SPONSORED SUN SENTINEL Former Miami Dolphins defender dies at age 48 DAVE HYDE SUN SENTINEL ‘The Talk’ cancels shows amid investigation surrounding Sharon Osbourne comments ALEX STEDMAN, VARIETY CHICAGO TRIBUNE Donald Trump’s Inauguration Day letter to Joe Biden: An exclusive (very real) draft What Happened to the Homecoming Queen? Check Here for Free CLASSMATES.COM | SPONSORED People On Medicare Are Getting A Big Surprise This March MEDICAREPLAN | SPONSORED SUN SENTINEL Three women charged in attack on Popeyes employee that was caught on video WAYNE K. ROUSTAN SUN SENTINEL A man tried to apologize to a 23-year-old woman he is accused of molesting when she was a little girl. Instead, he was arrested WAYNE K. ROUSTAN ADVERTISEMENT by TaboolaSponsored LinksYou May Like The Poorest States In The US, Ranked MoneyWise.com Windows Users Don't Forget To Do This Before Sunday (Do It Now) Bright Life Are You Over 40? This game is a must - No Install Raid Shadow Legends If You Like to Play, this City-Building Game is a Must-Have. No Install. Forge Of Empires MOST READ • BUSINESS Is Biden to blame for rising gas prices? MAR 15, 2021 Is Biden to blame for rising gas prices? Claim your free money: The IRS owes millions to the unemployed MAR 17, 2021 Claim your free money: The IRS owes millions to the unemployed The red-hot housing market means you’re probably in for a tax increase 11:13 AM The red-hot housing market means you’re probably in for a tax increase CONNECT TRIBUNE PUBLISHING Chicago Tribune The Baltimore Sun The Morning Call of Pa. Daily Press of Va. The Daily Meal New York Daily News Orlando Sentinel Hartford Courant The Virginian-Pilot Studio 1847 COMPANY INFO Contact the Newsroom Place an ad Privacy Policy Manage Web Notifications Newspaper Online Careers Terms of Service Feedback Copyright © 2021, South Florida Sun-Sentinel
Tuesday, March 16, 2021
Purpose of Blog
Search This Blog
Featured Post
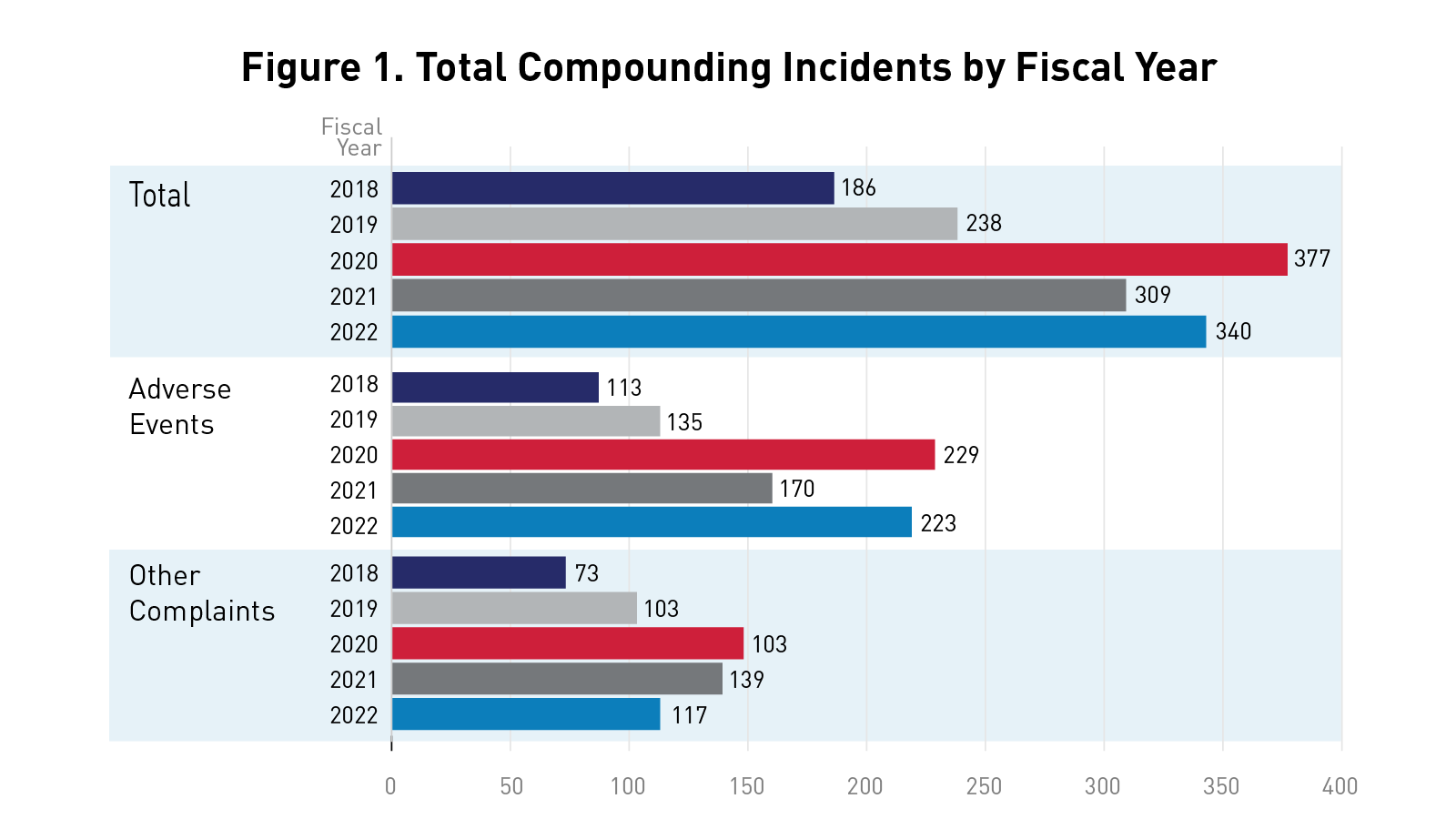
Essential Information: FDA Mitigating Risks of Compounded Drugs Through Surveillance
Mitigating Risks of Compounded Drugs Through Surveillance FDA’s Compounding Incidents Program aims to help protect the public against poor...